
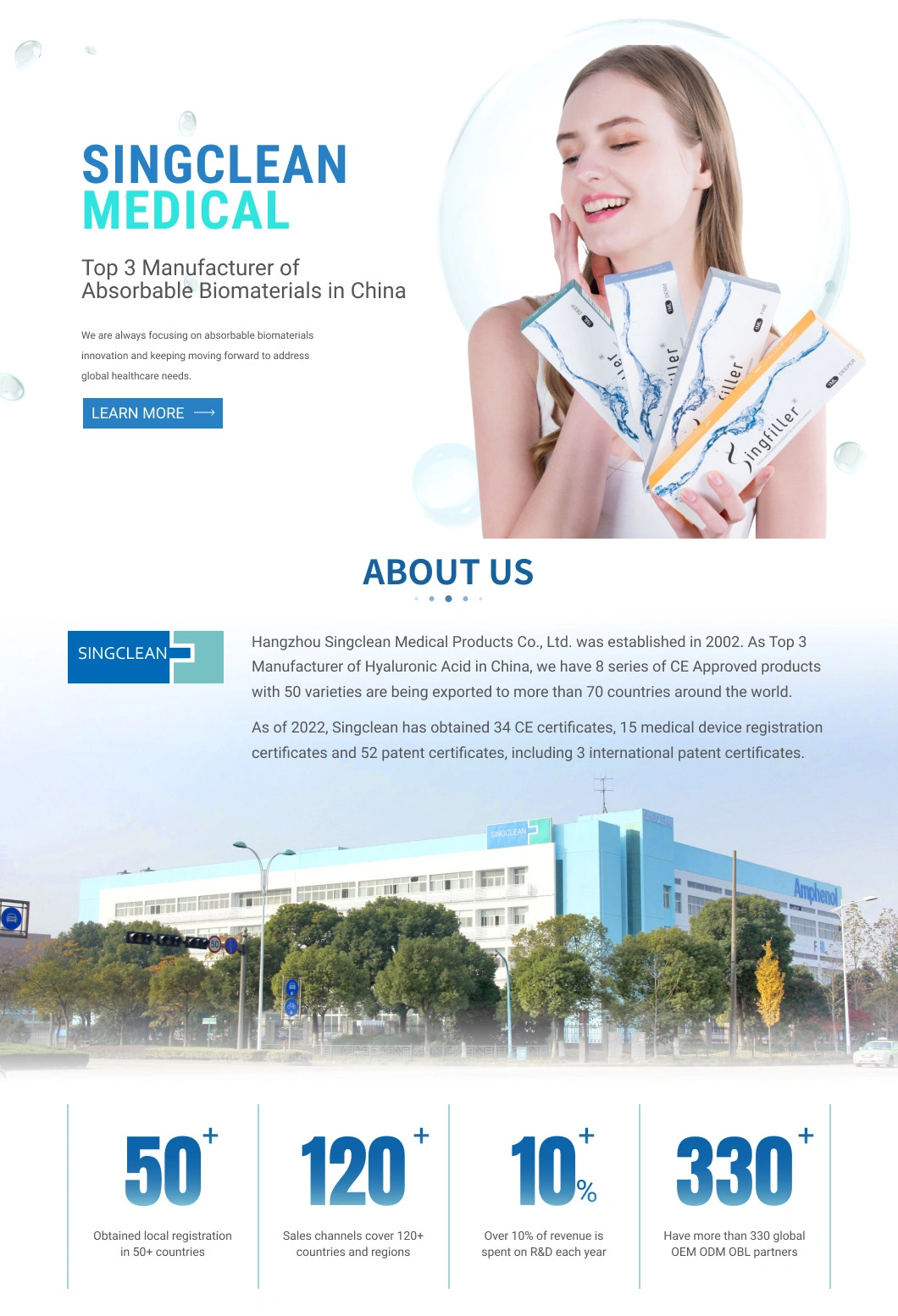
Singderm Hyaluronic Acid Injection Dermal Filler with 0.3% Lidocaine Cosmetics Aesthetic Filler for Plastic Surgery
Overview Singderm Lidocaine Cross-linked Hyaluronic Acid Injection Filler 10ml Key Words: Hyaluronic Acid, dermal filler
Send your inquiryDESCRIPTION
Basic Info
| Model NO. | Singderm |
| Syringe | Bd 10.0 Prtc |
| Function | Anti-Aging |
| Gender | Unisex |
| Shelf Life | 2 Years |
| Model No | Singderm |
| Color | Transparent |
| Material | Hyaluronic Acid |
| Certification | FDA, QS, CCC, RoHS, ISO, CE, Cfda |
| Transport Package | Prefilled Syringe in Blister |
| Specification | 1ml 10ml |
| Trademark | Singderm |
| Origin | China |
| HS Code | 30067000 |
| Production Capacity | 1, 000, 000PCS |
Product Description
Singderm Lidocaine Cross-linked Hyaluronic Acid Injection Filler 10ml
Key Words: Hyaluronic Acid, dermal filler, facial hyaluronate acid, cosmetic products, plastic surgery, high quality, good effect, Anti Aging, Nasolabial folds, lip filler
Product Description
Singderm® is a sterile, biodegradable, nonpyrogenic, viscoelastic, clear, colorless, homogenized gel implant. It consists of modified hyaluronic acid (HA) produced by bacteria, formulated to a concentration of 24mg/ml and 0.3% lidocaine in a physiologic buffer.
| Brand | Singderm |
| Composition | 24mg/ml (HA) with 0.3% Lidocaine |
| Recommended indications | Thin superficial lines, moderate wrinkle, deep facial wrinkles and folds, minimally invasive orthopedics and soft tissue flling, like reduce wrinkles, lip filer, hump nose, chin fullness. |
| Injection Depth | Upper and middle part of Dermis |
| Volume of Syringe | 1 mI/20ml |
| Storge Temperature | 2.up to 30 °C (35.6 °F --86°F) |
| Duration | 9-12months |
| Feature | Longer lasting, no pain |
Our Advantages
| 1). Effectivea. good volumeHigh viscoelasticity, not easy to shift deformation caused by external force. and strong support, keep good volume.b. Long durationThe high concentration of 24mg / ml and crosslinked polymer network structure are not easy to be degraded, and can last more than 12 months. | 2). Safetyc. Our Material is approved by American FDA and European EDQM.d. With the terminal sterilization, can ensure the sterility of 106 level ; Use BD hylokTM syringe, with smooth injection and good sealing. Multiple process ensures safety.e. Good biocompatibilityStreptococcus fermentation, Non - animal origin , osmotic pressure and pH value close to human body, greatly reducing the probability of swelling. | 3). Soft and natural, relaxed injection experiencef. Singderm uses single-phase cross-linking technology, is smooth and uniform, no particle foreign body feeling, soft texture.g. It contains 0.3% lidocaine, which can effectively reduce the pain caused by injection. |
Applications
1) Nasolabial folds2) Nose3) Chin4) Lip
Recommended Use
Real Feedback from Users
Main Products
Company Profile


Related Products
-
![Disposable Medical Supplies New Nucleic Acid Detection Kits Detection Reagents Approved CE]()
Disposable Medical Supplies New Nucleic Acid Detection Kits Detection Reagents Approved CE
-
![Hot Selling Safe Korea Ultra V Lifting 4dblunt Cannula Pdo Lifting Mono Thread]()
Hot Selling Safe Korea Ultra V Lifting 4dblunt Cannula Pdo Lifting Mono Thread
-
![Beauty Equipment Diode Laser Laser Treatment Device Nail Fungus Laser Treatment]()
Beauty Equipment Diode Laser Laser Treatment Device Nail Fungus Laser Treatment
-
![Free Logo Hyaluron Injectable Pen Filler Hyaluronic Acid Hyaluronic 0.3/0.5 Ampoule Acid Injection Cross Linked]()
Free Logo Hyaluron Injectable Pen Filler Hyaluronic Acid Hyaluronic 0.3/0.5 Ampoule Acid Injection Cross Linked











